MCCMCC Microbiology Lab Report
Description
Lab 4
Specimen Preparation and Staining
Introduction: Connecting Your Learning
This laboratory exercise will introduce several types of staining techniques for viewing bacteria under a microscope.
| Multimedia Resources |
None |
| Required Assignments |
Lab 4 Assignment |
Focusing Your Learning
Course Competency
- Identify microorganisms in the laboratory, emphasizing bacteria.
As you work through this lab, consider the objectives below.
Lab Objectives
By the end of this lab, you should be able to:
- Describe procedures for preparing specimens for observation under the microscope.
- Identify bacteria using various staining techniques.
- Describe the differences in types of stains and uses for each stain.
Background Information
Bacteria are not readily examined microscopically in a living, unstained state. But examination of living microorganisms can be useful to observe cell activities, such as motility. It is also useful to observe the natural sizes and shapes of the cells because heat fixation and chemicals during the staining process cause some degree of cellular distortion.
The primary means of viewing bacteria in a living state is the hanging drop procedure. Living organisms can be viewed using the hanging drop procedure. This procedure is used to view cell shape and size as well as motility. True motility is self-directed by the organism, while Brownian movement is a vibratory movement of the cells due to their bombardment by water molecules in the suspension.
To make a hanging drop slide:
- Using a depression slide, spread a ring of petroleum jelly around the concavity of the slide.
- Place a loopful of bacterial culture in the center of the coverslip.
- Lower the depression slide with the concavity facing down onto the inoculated coverslip. Press gently to seal.
- Turn the hanging-drop slide over so that the coverslip is on-top and the culture drop adheres to the coverslip.
Preparation of a hanging drop slide:
 Creative Commons https://upload.wikimedia.org/wikipedia/commons/d/d… by Macedo, CC BY-SA 4.0
Creative Commons https://upload.wikimedia.org/wikipedia/commons/d/d… by Macedo, CC BY-SA 4.0
Visualization of living microorganisms is difficult, not only because they are small but also because they are transparent and practically colorless. To study their properties and to divide microorganisms into specific groups for diagnostic purposes, biological stains in conjunction with microscopy have become major tools in the microbiology lab.
Bacterial smears must be prepared prior to performing any staining technique. Smears are made from broth cultures or from cultures of a solid medium. The technique varies for broth and solid media.
With broth cultures, one or two loopfuls of the broth should be applied to the center of the slide using a sterile inoculating loop and spread evenly over a dime-size area of the slide. Allow the slides to air dry.
With cultures from solid media, the cultures must first be diluted. This is done by placing one or two loopfuls of water on the center of the slide where the cells will be diluted. Transfer of the cells requires the use of a sterile inoculating loop or needle. Only the tip of the loop or needle should touch the culture to prevent too many cells from being transferred. Suspension is accomplished by spreading the cells in a circular motion in the drop of water on the slide with the loop or needle. Then the sample should be heat fixed.

The preparation of a bacterial smear varies by if the specimen is from a broth culture or a solid culture.
by Microbiology A Laboratory Manual. 6th Edition.
Heat fixation is performed to prevent the bacterial smear from washing away during the staining procedure. Heat fixation can be performed by the rapid passage of the air-dried smear over the flame of a Bunsen burner.
Once the bacteria have been fixed onto a slide, the slide needs to be stained for easier viewing. Several types of stains are used in the microbiology laboratory.

a) A specimen can be heat-fixed by using a slide warmer like this one. b) Another method for heat-fixing a specimen is to hold a slide with a smear over a microincinerator.
 Creative Commons https://openstax.org/books/microbiology/pages/2-4-… is licensed under CC BY 4.0.
Creative Commons https://openstax.org/books/microbiology/pages/2-4-… is licensed under CC BY 4.0.
THE CHEMISTRY OF STAINING:
Most stains used in microbiology are synthetic aniline dyes (compounds derived from benzene, a coal tar derivative). These dyes are usually salts, although a few are acids or bases composed of two charged ions (the positively charged ion is the cation, and the negatively charged ion is the anion). Either one of the ions can be the chromophore, which is the part of the molecule that is brightly colored. For example:
If the chromophore is a positive ion like methylene blue, the stain is considered a basic stain. Note that most cells typically have negatively charged cell walls, so the positive chromophores in basic dyes tend to stick to most cell walls (given their negative charges). As such a basic stain is the most frequently employed type of microbiological stain. But if the chromophore is a negative ion, then it is an acidic stain.
Most bacteria are stained when a basic stain permeates the cell wall and adheres by weak ionic bonds to the negative charges of the bacterial cell. A few of the basic dyes one encounters in this course are methylene blue, crystal violet, basic fuchsin, and safranin.
Acidic dyes, such as nigrosin, are generally repelled by bacteria because of the negative charges on their chromophores. As such, acidic stains are used to color the background while leaving the cells colorless.
SIMPLE STAINING PROCESSES:
In simple staining, the bacterial smear is stained with just one single dye. The purpose of simple staining is to simply allow the morphology and arrangement of bacterial cells to be seen more clearly. Simple stains use a single stain to color bacterial cells so that their size, shape, and arrangement can be observed. The most commonly used stains are methylene blue, crystal violet, and carbol fuchsin. The stain is applied to a fixed smear and then rinsed off with water. Simple stains are used when the organism is in a pure culture, which means that the culture contains only one organism. A simple stain that stains the bacterial cells themselves is a direct stain, and a simple stain that stains the background but leaves the bacteria unstained is a negative stain.
Negative stains use a single stain to color the background around cells. The stains will not penetrate the cells. Therefore, the unstained cells are easily seen against the colored background. Heat fixation is not required for negative stains as heat and chemicals will not distort cell shape and size. It also allows the microscopic observation of cells that do not easily stain, such as spirilla and spirochetes.

Simple Stains Chart
 Creative Commons https://openstax.org/books/microbiology/pages/2-4-… is licensed under CC BY 4.0.
Creative Commons https://openstax.org/books/microbiology/pages/2-4-… is licensed under CC BY 4.0.
DIFFERENTIAL STAINING PROCESSES:
Differential staining requires the use of at least three chemical reagents that are applied sequentially to a heat-fixed smear.
- The first reagent is the primary stain; its function is to impart its color to all cells.
- To establish a color contrast, the second reagent used is the decolorizing reagent. The decolorization reagent may or may not remove the primary stain from the entire cell based on the chemical composition of cellular components.
- The final reagent, the counterstain, has a contrasting color to that of the primary stain. Following decolorization, if the primary stain is not washed out, the counterstain cannot be absorbed, and the cell or its components will retain the color of the primary stain. If the primary stain is removed, the decolorized cellular components will accept and assume the contrasting color of the counterstain.
In this way, cell types or their structures can be distinguished from each other on the basis of the stain that is retained. Differential staining can be used when the culture is not a pure culture. If two or more organisms are present in the culture, differential staining aids in the differentiation and identification of the multiple organisms.
Gram Stain
The most important differential stain used in microbiology is the Gram stain. It divides bacterial cells into two major groups, Gram-positive and Gram-negative, which makes it an essential tool for classification and differentiation of microorganisms. The Gram stain reaction is based on the difference in the chemical composition of bacterial cell walls and the ability of the bacterial cell wall to retain crystal violet during staining. Most bacteria possess a cell wall that contains either a thick peptidoglycan layer or a thin peptidoglycan layer with an additional lipopolysaccharide layer. Gram-positive cells have a thick peptidoglycan layer, and Gram-negative cells have a much thinner peptidoglycan layer while also being surrounded by outer lipid-containing layers.
The reagents used for the Gram stain are crystal violet, Gram’s iodine, ethyl alcohol, and safranin. Crystal violet is the primary stain. It stains cells purple. Gram’s iodine is the mordant. A mordant increases the cells affinity for the primary stain and forms a crystal violet-iodine complex that prevents the dye from being removed; the crystal violet is trapped inside the cell wall of gram-positive bacteria. But in gram-negative bacteria, the decolorizing agent removes the crystal violet-iodine complex. Ninety-five percent ethyl alcohol is the decolorizing agent. Safranin is the counterstain, which stains cells pink-red.

The 4-Step Gram Stain Process
 Creative Commons https://openstax.org/books/microbiology/pages/2-4-… is licensed under CC BY 4.0.
Creative Commons https://openstax.org/books/microbiology/pages/2-4-… is licensed under CC BY 4.0.
Bacteria with a thick peptidoglycan layer retain the primary stain, crystal violet, during decolorization and are referred to as Gram-positive. Gram-positive bacteria appear purple when viewed under the microscope.
Bacteria with a thin layer of peptidoglycan and an additional lipopolysaccharide layer lose the primary stain during decolorization and retain the counterstain safranin. These bacteria are referred to as Gram-negative. Gram-negative bacteria appear pink-red when viewed under the microscope.
Reactions to Gram Staining should be identified as either Gram-positive or Gram-negative.
In a known pure culture in which there is only one organism, that organism should be stained only one color. But human error during decolorizing can impact the results of the Gram stain procedure. If the culture is a pure culture, but the stain reaction appears to show both gram-positive and gram-negative reactions, then the staining technique was performed poorly. Either the organism was over decolorized, and a gram-positive organism is staining as a gram-negative organism; or, the organism was under-decolorized, and a gram-negative organism is staining as a gram-positive organism. In addition, older bacterial cells may sustain damage to their cell walls over time; this can cause some gram-positive cells to appear gram-negative even if the species is truly gram-positive. For this reason, it is important to use fresh bacterial samples when gram staining.
If the culture is not a pure culture, and there are two or more organisms present in the smear, then it becomes important to view morphologies (cell shapes) and stain reactions (by color) to further differentiate the organisms.
Micrograph of gram-positive cocci mixed with gram-negative rods
 Creative Commons
Creative Commons 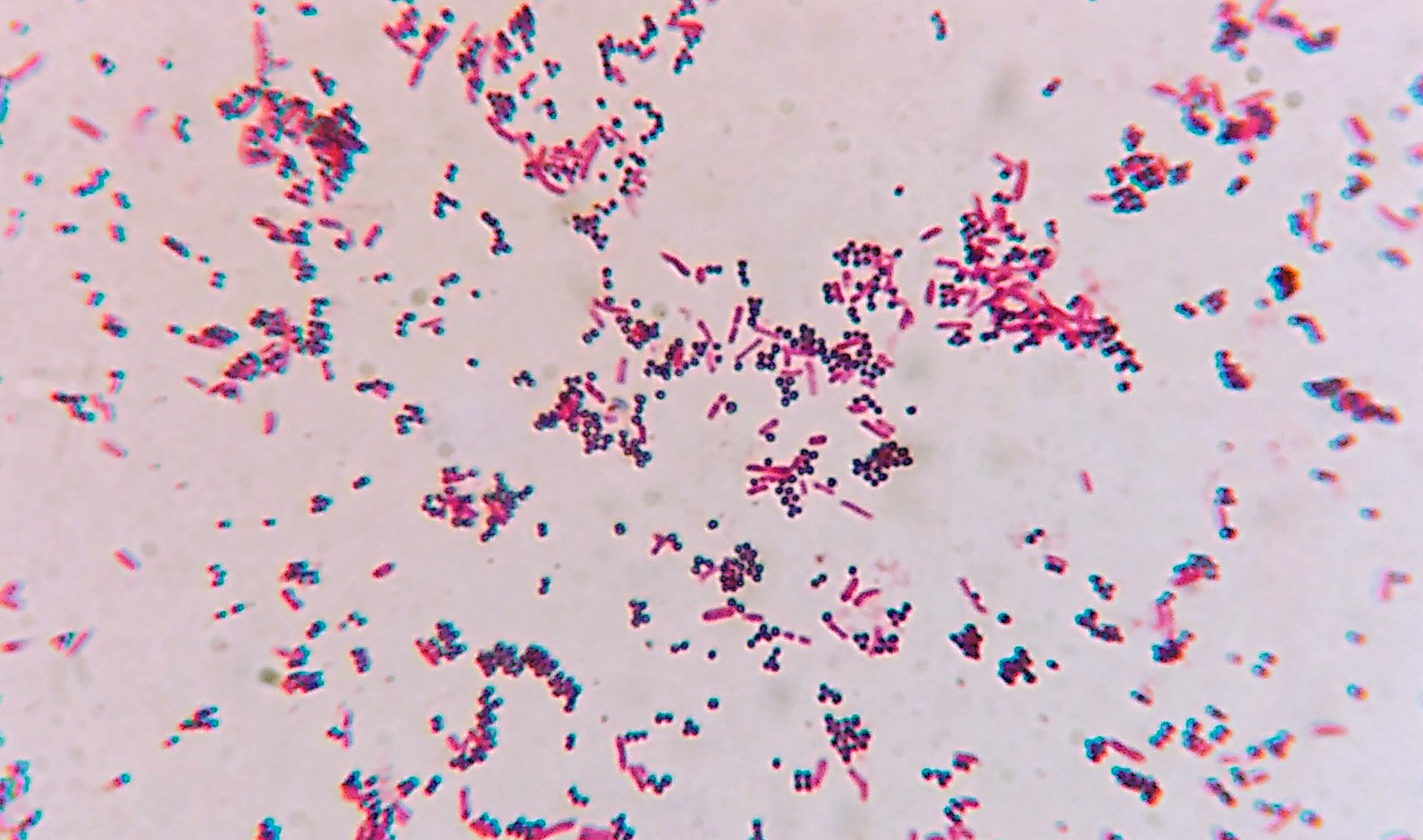
by Microrao, CC BY-SA 4.0
Acid-fast Stain
While the majority of bacterial organisms are stainable by Gram staining, a few, particularly the Mycobacteria, which cause tuberculosis, are resistant and can only be visualized by the acid-fast method of staining. The acid-fast stain distinguishes different types of bacteria based on the wax content of their cell wall.
The characteristic difference between the Mycobacteria and other microorganisms is the presence of a thick, waxy wall that makes penetration by stains extremely difficult. The acid-fast stain uses three different reagents. Carbol fuchsin is the primary stain, and it stains all cells pink/red. The decolorizer is acid-alcohol. On application of acid-alcohol, acid-fast cells will be resistant to decolorization and will stay a red color. For non-acid-fast organisms, the primary stain is removed during decolorization. The counterstain is methylene blue, and will stain the unstained, non-acid-fast cells a blue color.
The Acid-fast Staining Process
 Creative Commons https://upload.wikimedia.org/wikipedia/commons/1/1… by Elizabeth Gray, CC BY-SA 4.0
Creative Commons https://upload.wikimedia.org/wikipedia/commons/1/1… by Elizabeth Gray, CC BY-SA 4.0
Bacteria with high wax content, which will not readily stain with the Gram stain, retain the primary stain carbol fuchsin when decolorized with acid-alcohol. These bacteria will appear pink/red and are called acid-fast. Bacteria with low wax content in their cell wall lose carbol fuchsin when decolorized and then take up the counterstain methylene blue. These bacteria appear blue and are called non-acid-fast.
Reactions to Acid-fast Staining can be either Acid-Fast when the pink/red cells are seen or Non-Acid-Fast when the cells appear blue.

Pink red rods of the Acid-fast bacilli Mycobacterium tuberculosis in sputum smear
 Creative Commons
Creative Commons 
by Microrao, CC BY-SA 4.0
SPECIAL STAINS:
Special stains can also be used for the visualization of specific bacterial cell structures. Three stains are commonly done in the microbiology lab: endospore stain, capsule stain, and flagella stain. Detecting these structures is an important step in their identification. Historically, staining techniques were used for detection; currently, most of the fine structural details are examined using an electron microscope.
Endospore Stain
During growth, some bacteria form an endospore within the cell and release it upon death of the cell. A free spore can survive in extreme environments. In favorable conditions, a spore can germinate to once again yield a vegetative cell.
The endospore stain is performed to visualize the spores in Cl
Get your college paper done by experts
Do my questionPlace an order in 3 easy steps. Takes less than 5 mins.





Leave a Reply
Want to join the discussion?Feel free to contribute!